New Study Explores Novel Antibody-Lectin Chimeras (AbLec) to Disrupt Tumor Immune Evasion
Researchers from the University Medical Center Schleswig-Holstein, Campus Kiel, contributed to a new international study led by teams at Stanford and MIT in the United States, which introduces antibody lectin chimeras as a promising strategy to counter tumor immune evasion and strengthen cancer immunotherapy.
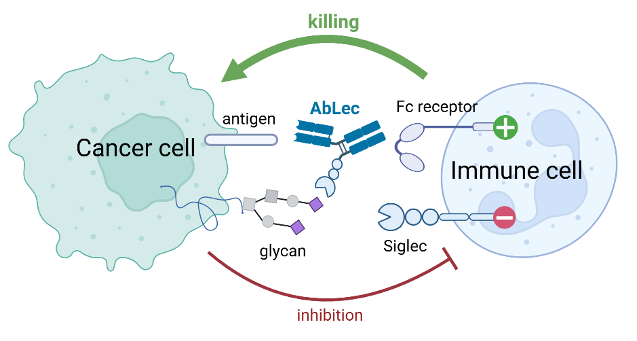
Immunotherapies have opened new possibilities in cancer treatment, but there are still patients that do not respond adequately to this therapy option, in part because tumors use sugars, known as glycans, on their surface to evade immune attack. These glycans can interact with inhibitory receptors, called Siglecs, on immune cells and suppress immune responses.
A new study, just published in Nature Biotechnology, presents a novel strategy to interfere with these sugar-based interactions. A multi-institutional research team developed antibody-lectin chimeras, called AbLec. These engineered molecules combine a conventional antibody that guides them specifically to tumor cells with a lectin domain that binds to tumor cell glycans. This structure allows AbLec to block inhibitory signals which cancer cells use to protect themselves from immune cell attack and at the same activate these immune cells via their antibody part.
In preclinical in vitro and in vivo models, AbLec restored immune activity, performed better than existing therapies and also showed potential when used alongside established checkpoint inhibitors. The approach can be adapted to target various types of tumors and immune cell interactions, making it a flexible tool for cancer therapy. A spin-off company intends to bring this innovative approach into clinical trials within the next 2-3 years.
The study was led by researchers at MIT and Stanford, including Dr. Jessica Stark and Prof. Carolyn Bertozzi, who is a pioneer in glycobiology and Nobel laureate in Chemistry. From the University Medical Center Schleswig-Holstein, Campus Kiel, CATCH ALL researchers Dr. Marta Lustig and Prof. Thomas Valerius (P6) contributed to the findings. The goals are to expand the AbLec platform to improve myeloid effector cell recruitment across different cancers. In addition, she aims to further improve the molecules by Fc engineering, thereby nurturing the collaboration with the groups at MIT and Stanford.
We congratulate our P6 colleagues on their involvement in this innovative work that opens new possibilities for overcoming immune resistance in cancer.
Original publication: https://www.nature.com/articles/s41587-025-02884-6
Press release MIT: https://news.mit.edu/2025/new-immunotherapy-approach-could-work-many-types-cancer-1216


